
Objective response (OR)
The measure for tumor shrinkage
OR is defined simply as tumor shrinkage and is a biomarker for treatment effect in phase II screening trials.1 Clinical trials in advanced-cancer settings are increasingly basing evaluation of clinical endpoints, such as time to disease progression, on anatomical measurement of tumor size.1

A baseline measurement of overall tumor burden is required for future progression assessments and serves as a comparator for subsequent measurements.1 Measurable disease at baseline should be used as inclusion criteria for protocols that assess objective tumor response as a primary endpoint.1
Measurable tumor lesions can be accurately measured, and the longest diameter is recorded.1 The longest diameters for all non-nodal target lesions will then be added to calculate the baseline sum of diameters, which is used to evaluate the OR.1
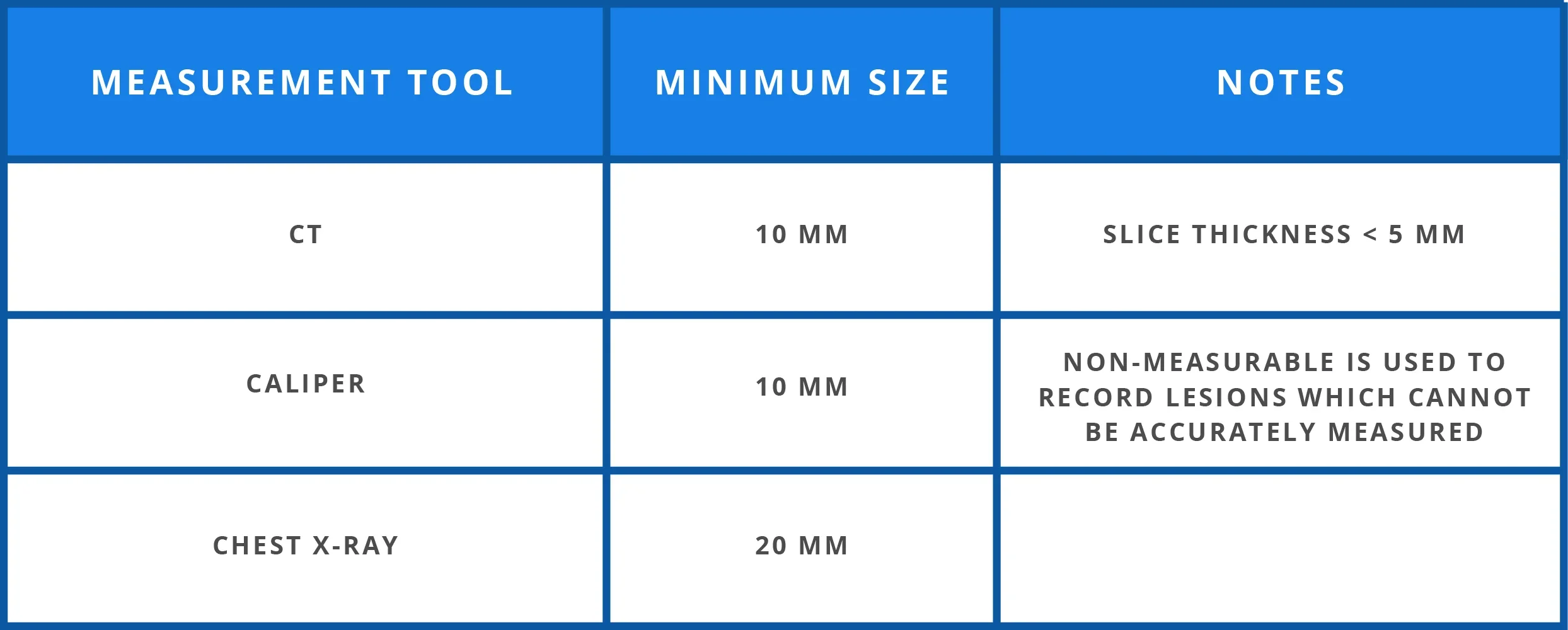
Measurability of tumour at baseline1

A maximum of 5 target lesions or 2 lesions per organ are chosen at baseline for their size (lesions with the longest diameter), representativity of all involved organs and reproducible repeated measurements.1
Two hypothetical examples of tumor shrinkage2
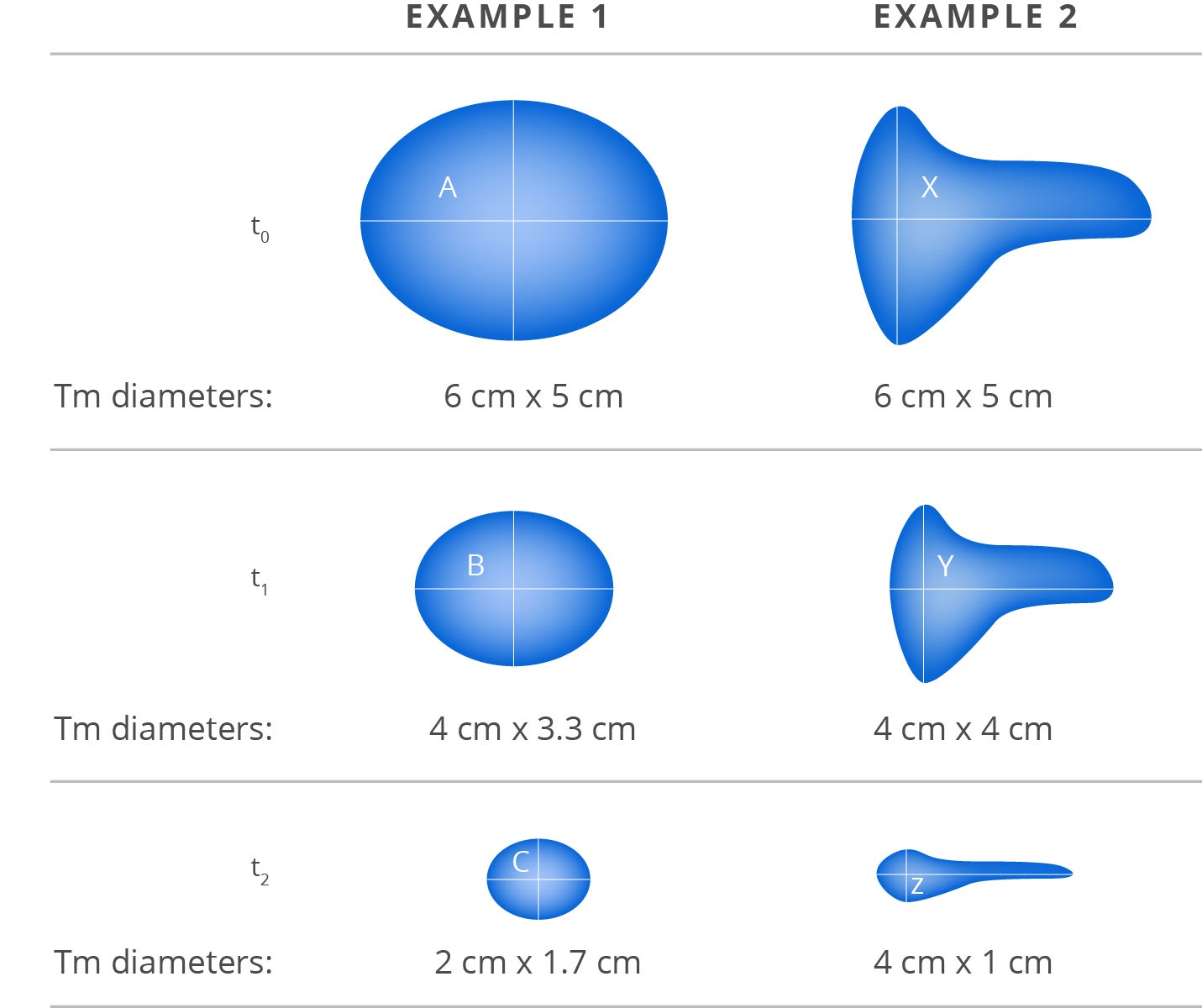
Adapted from Aykan et al. 2020.2 Example 1: Spheroid mass, symmetric (proportional) shrinkage; Example 2: Irregular mass, asymmetric shrinkage. t0: Time point baseline; t1: Time point-1; t2: Time point-2; A, B, C: Spheroid mass; X, Y, Z: Irregular mass.2
CT: computed tomography; OR: objective response; PFS: progression-free survival.
- Eisenhauer EA, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009 Jan;45:228–47.
- Aykan NF, Özatlı T. Objective response rate assessment in oncology: current situation and future expectations. World J Clin Oncol. 2020;11:53–73.